As well as improving the lives of cancer patients whose needs are not currently being met, these impacts would benefit the economy and society as a whole. There is currently a once-in-a-generation opportunity to optimize regulatory timelines in order to improve time to patient access for cancer patients across Europe.
Our latest report in the series Every Day Counts can be downloaded here.
A NEED FOR CHANGE
The journey for a new medicine from laboratory to patient is long and for cancer patients, every day counts. And while research development, clinical trials and approval all take time for good reason, the current timelines for the essentially administrative processes between CHMP opinion and EC decision come at a real cost.
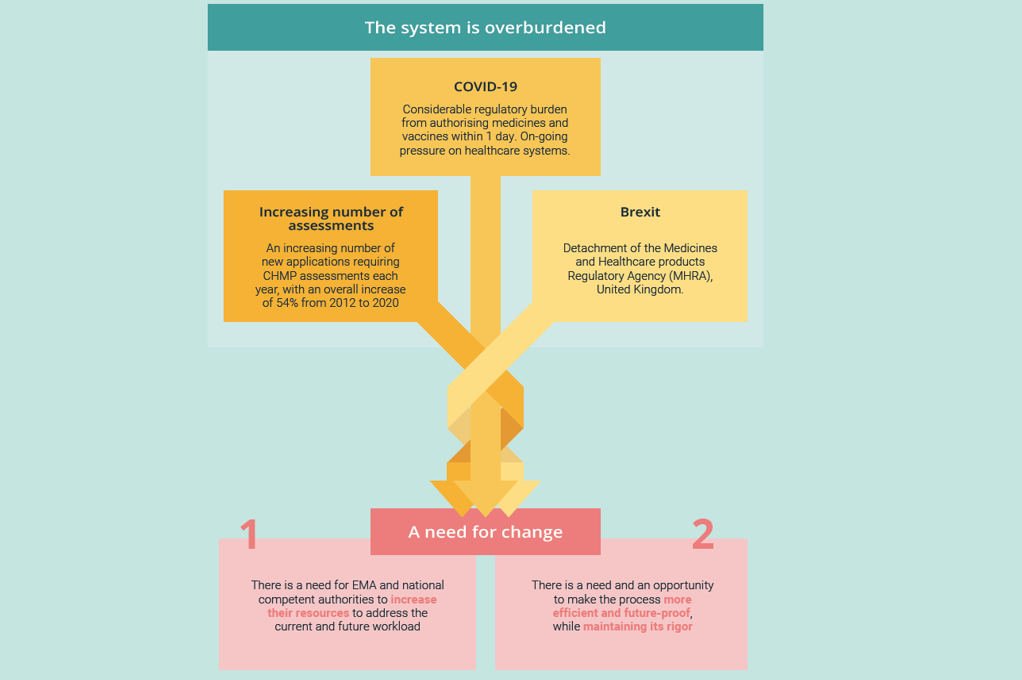
As well as offering real benefits to patients and other stakeholders, optimizing these processes gives us a real opportunity to increase efficiency and future-proof a system which is currently overburdened as a result of challenges including Brexit, COVID-19, and the ever-increasing pipeline of assessments of new therapies.

A MOMENTUM FOR CHANGE
With a maximum official duration of 67 days (and a range of 33-198 days in practice), this administrative process final CHMP opinion and the EC decision represents only a small part of a medicine’s journey from the laboratory to patients. But it represents a significant and achievable opportunity for improvement.
During the COVID-19 pandemic, the process was expedited in less than a day to hasten the conditional approval of the first COVID-19 vaccines. Though this was an exceptional case and deserved an exceptional response, the fact this was achieved does suggest it is possible to shorten timelines for other approvals significantly. Furthermore, it is possible to streamline this process without diminishing either the quality or the rigor of the CHMP’s scientific review, which must remain sacrosanct.
The time is also right for change. The EC is currently considering changes to pharmaceutical legislation as part of the European Union’s pharmaceutical strategy. This strategy is only revisited every generation, giving us an almost unique opportunity: to seize on recent learnings to improve the lot of millions of patients for decades to come.
ONE DAY THAT CHANGED EVERYTHING
The demand for COVID-19 vaccines across Europe and the world in 2020 provided the impetus for the EMA and EU Member States to accelerate the formal process and show how quickly they could act together when required. The process between final CHMP opinion and the EC decision to grant a conditional marketing authorisation was completed within just one day.
This was, of course, an exceptional event in response to an unprecedented crisis. It is not feasible, realistic or even necessarily desirable to adopt the approach taken to expedite the approval of the vaccines. But it is also important to remember that, in the same way it was critical to access COVID-19 vaccines as quickly as possible, oncology patients have no time to lose. We should learn lessons, design strategies and implement learnings from the pandemic experience to accelerate normal timelines for innovative oncology treatments.
THREE ROUTES TO FASTER APPROVALS
Vintura, working in collaboration with a group of more than 30 organizations which together represent all relevant stakeholders, has established a clear and common understanding between those stakeholders of the steps involved in the process between final CHMP opinion and the EC decision to grant a marketing authorisation [read more details in blog 2]. We used this analysis to identify potential opportunities to reduce timelines in order to improve the value the approvals process can deliver to patients and to society.
This work, which was commissioned by the EFPIA Oncology Platform, has resulted in several strategic, concrete solutions to optimise and accelerate the process between CHMP opinion and EC decision. These potential solutions were assessed according to their impact and feasibility, and the following solutions were identified as offering the greatest potential benefit. It is important to note that they can all be used in combination.
- Conduct the decision-making phase in parallel to the linguistic phase. This would enable the granting of Marketing Authorisation 12 days earlier
- Increase the use of digital tools during the linguistic phase, which could shorten this phase by 10 days
- Provide an opportunity to shorten the written procedure in cases where Member States foresee no objections. This would shorten the decision-making phase by 15 days
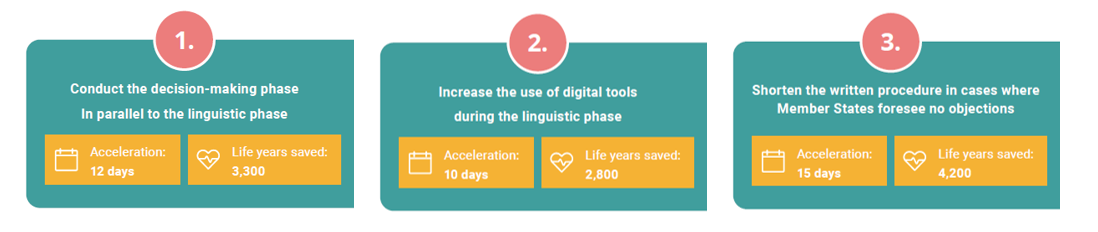

Combining these solutions could save thousands of years of potential life across the EU for oncology patients alone. But this is only the beginning of their potential. The proposed new process would apply to all other products that receive a European marketing authorisation by means of the centralised procedure – which would have a huge impact for a significant proportion of the European population. Together, let’s change the current process by making use of this once-in-a-generation opportunity offered by the Pharmaceutical Legislation review.
THE BIGGER PICTURE
The 67-day process that is the focus of this blog and the latest Every Day Counts report represents only a small piece of the entire journey of a medicine from laboratory to patient [read more details in our blog 1]. If stakeholders were to address it as part of a joint and comprehensive effort to align and accelerate the whole European system of Marketing Authorisation, Market Access and Patient Access, major gains in terms of life years, quality of life and productivity could be made across the EU.
The opportunity is ours. Together, we should seize it.
Let’s discuss
Inspired to share your thoughts? Or would you like to learn more on how to work together effectively with multiple stakeholders to shorten times to patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Here are all the articles in the “Every day counts” series
- White paper release: Every day counts – Improving time to patient access to innovative oncology therapies in Europe
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access
- Blog: Bringing new cancer treatments to Europe: the challenge of making it across all three access milestones
- White paper release: Every day counts –The impact of COVID-19 on patient access to cancer care in Europe.
- Blog: How COVID-19 has affected European patients’ access to cancer care, and what we can learn from the crisis
- Blog: COVID-19 has hurt our ability to reduce cancer deaths
- Blog: 6 learnings from the COVID-19 crisis to make cancer care delivery in Europe more resilient to future challenges
- White paper release: Every day counts – Improving regulatory timelines to optimise patient access to innovative oncology therapies in Europe
- Blog: Optimising European regulatory timelines can save thousands of years of potential life
- Blog: Save time. Save lives.


