The last few decades have seen the number of cancer deaths grow more slowly than the number of cancer diagnoses across Europe. This welcome trend is the result of pharmaceutical innovations, more effective prevention, better screening and radiotherapy, and new surgical techniques (Hofmarcher, et al., 2019).
The arrival of the COVID-19 pandemic in early 2020 has put this trend under severe strain. In early 2021 countries across Europe have seen excess deaths not only as a direct result of COVID-19, but also as a consequence of the indirect strain the pandemic has caused to healthcare systems in general (Whiting, 2020; Woolf et al., 2020). One of these strains has been increased delays in patient access to cancer care, which added to the 10 systemic factors which were already delaying patient access to oncology prior to the crisis.
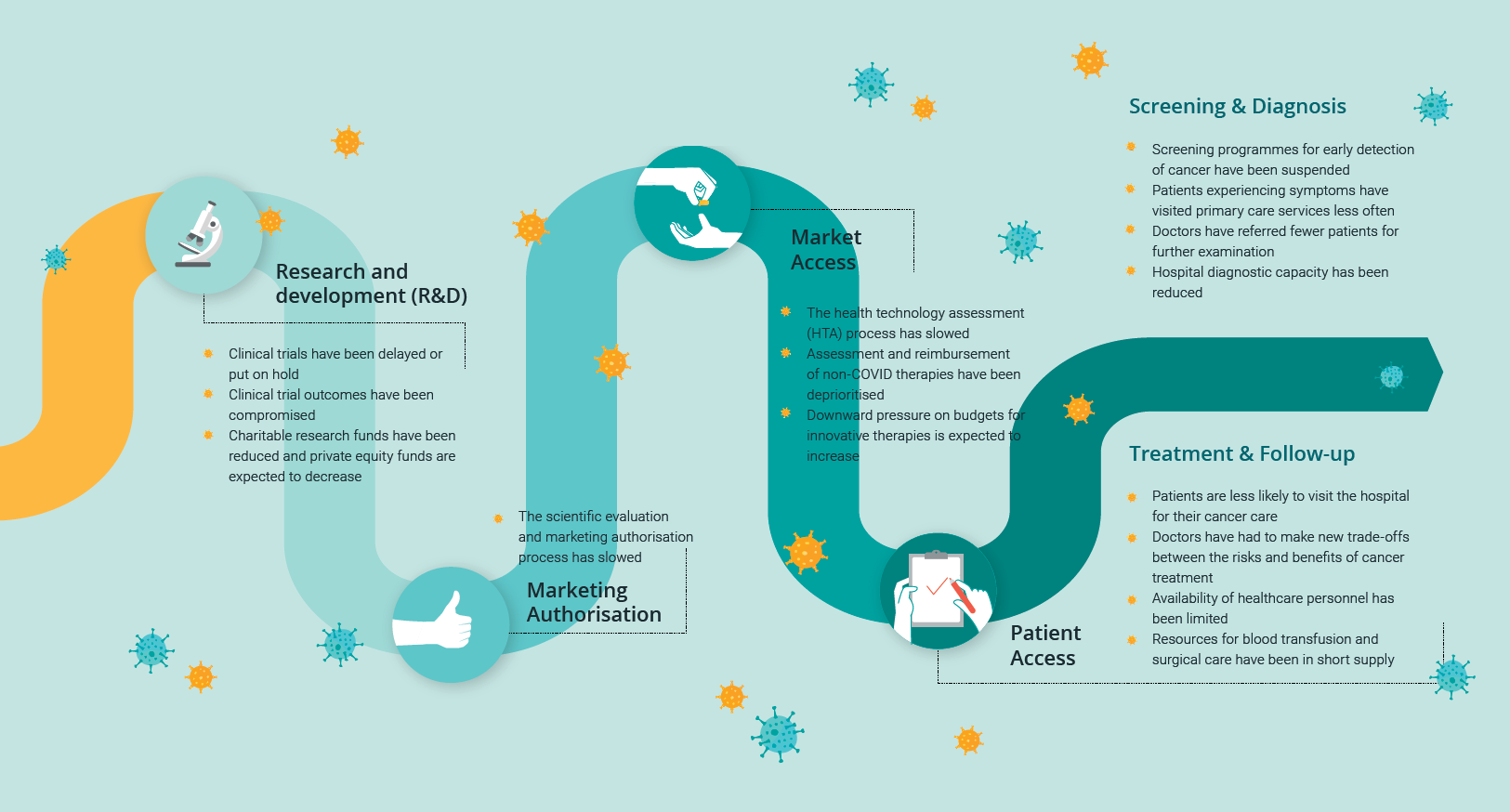
Together, these disruptions have impacted all four milestones identified in our original report as necessary in order to ensure access to innovative oncology therapies:
- Research & Development
- Marketing Authorization
- Market Access
- Patient Access

research & development severely impacted
The continuity of the research and development process has been severely impacted by the COVID-19 pandemic in a number of ways.
- In May 2020, a global survey found 19% of brain tumour patients could no longer participate in clinical trials (Voisin et al., 2020), while in July 2020 over a fifth of global oncology trials were halted due to the pandemic. This impact was felt severely by patients with breast, prostate, and lung cancer (Tempest & Taylor, 2020).
- In March and April 2020, a survey among seven investigators leading trials in Europe indicated that patient enrolment in active oncology clinical trials was negatively affected at the time of the survey, with only 14% of the institutions continuing to enroll patients at the usual rate (Upadhaya et al., 2020)
Perhaps more alarmingly, those trials which could continue often required significant changes in setup, which are highly likely to lead to difficulties in convincing stakeholders of the efficacy of promising therapies, as the cancellations of biopsies and imaging mean results demonstrating progression-free survival endpoints are based on patient reports rather than measurement of clinical outcomes.
The pandemic also had a negative effect on the funding of clinical trials. Venture capital funding is more difficult to secure during a recession (Lorgelly & Adler, 2020) and many charitable research funds have had to reduce their research expenditure considerably as fundraising opportunities had to be cancelled (Erasmus MC, 2020).
marketing authorisation slowed
Delays in the EMA marketing authorization process have occurred despite the dedicated taskforces set up to protect the body’s non-COVID-19 activities. The EMA has aimed to ensure the assessment and monitoring of cancer medicines is not disrupted (European Medicines Agency, 2020). Regulatory committees are, however, likely to face staffing challenges, as many members are clinicians, public health, or allied health professionals. There will also be one-off delays due to identifying, procuring, and implementing secure videoconferencing platforms. Patient participation in these committees may also have been negatively affected by the switch from physical to virtual meetings. (Lorgelly & Adler, 2020).
market access disrupted
The COVID-19 pandemic has impacted on the Market Access process in three different ways, risking the slowing of access to innovative oncology treatments for current patients and reducing the rate of reimbursement of innovative therapies for future patients.
The first impact is the slowing of the health technology assessment (HTA) process. These delays may lead to serious post-crisis backlogs which could prevent patients from accessing promising drugs as quickly as would otherwise be possible.
- In April 2020, the Ministry of Health in Poland announced their reimbursement list would not change until after the pandemic was over (APM International, 2020). There was a delay of two months as the country missed one bimonthly list update, after which the regular assessment process was restored.
The second factor is that the assessment and reimbursement of non-COVID therapies, including oncology therapies, have been deprioritized.
- EUnetHTA decided to deprioritize non-COVID-19 initiatives until the end of May 2021, as a result of some 23 EUnetHTA Rolling Collaborative Reviews (RCR) being performed for COVID-19 treatments (EUnetHTA, 2020).
Lastly, downward pressure on budgets for innovative therapies is expected to increase as European countries recover from the impact of the pandemic.
patient access restricted
According to the World Health Organization (WHO), 55% of countries reported disruptions to their cancer services due to the pandemic. Disruptions to screening, diagnosis, treatment, and follow-up are apparent in many European countries.
Screening & Diagnosis
- As of March 2020, the Netherlands halted national screening programs for breast, colorectal, and cervical cancer. Screening was continued during the summer period, but the number of invitations was lower than usual (Rijksinstituut voor Volkgsgezondheid & Milieu, 2020). For breast cancer screening, it was decided to reduce screening frequency from every two years to every three years, due to a shortage of health personnel which was exacerbated by the pandemic (Bevolkingsonderzoek Nederland, 2020).
- Screening numbers in Spain dropped dramatically: the number of colorectal cancer patients diagnosed based on screening dropped from 33.3% in March to June 2019 to 5.2% in the same period in 2020 (Suarez et al., 2020).
- At the start of the outbreak in England, urgent referrals for early diagnosis of suspected cancers decreased by 76% compared with pre-COVID-19 levels (Lai et al., 2020; Kampf & Kulldorff, 2021).
Treatment & Follow-up
- In the United Kingdom, appointments for (PET-)CT scanning were put on hold for three months during the initial phase of the pandemic. At the same time, infection control measures almost doubled the time required per diagnostic scan. Diagnostic capacity is thus likely to be a key bottleneck, leading to further delays once cancer services are resumed and backlogs start to be addressed (UK Lung Cancer Coalition, 2020, Tempest & Taylor, 2020).
- In France, cancer diagnoses decreased by 36% in April 2020 compared to the previous year (Santi & Pineau, 2020), while 18 Austrian cancer centers together recorded a 40% drop in breast cancer diagnoses between March and May 2020.
- In the areas most affected by COVID-19 in Italy, 38 to 51% of oncologists were reassigned to take care of COVID-19 patients (Indini et al., 2020).
- Seven comprehensive care centres in Europe reported that in April 2020 the number of cancer patients admitted fell by 20-30% (Van de Haar et al, 2020).
for cancer patients, every day counts
For cancer patients, early diagnosis is a key determinant of timely treatment and survival outcomes, so these delays are significant and worrying. Outcomes for cancer patients are likely to be negatively affected if the usual standard of care is delayed. (Whiting, 2020). If breast cancer is detected at stage I, the 5-year survival rate is nearly 100%. If detected at stage III it falls to 72% (John & Broggio, 2019). Lung cancer follows the same pattern, with one-year survival reaching 87.3% for stage I disease, but only 18.7% for stage IV (Hawkes, 2019).
In oncology, a treatment delay of four weeks is associated with a 6-13% increase in the risk of death (Hanna et al., 2020).
The exact magnitude of the effects of the pandemic on survival will only become apparent in the next few years. But following the first peak of the pandemic and its observed effect of delaying cancer diagnosis and treatment, researchers have already begun to make forecasts of the impact of these delays on patient survival rates. Figures vary depending on national context, the methods used, and the type of cancer. But all the studies paint a bleak picture.
In England, delays in diagnosis and treatment are expected to increase the number of cancer deaths from 5% to 17% (depending on cancer type) within 5 years of diagnosis (Marigne et al., 2020).
we must all learn from this crisis
Governments were forced, quite reasonably, from early 2020 to the present, to adopt drastic measures to address the pandemic. These measures were often to the detriment of cancer care. Health systems across Europe accelerated the implementation of digital healthcare and other innovations which mitigated the negative impact on cancer patients, and which achieved equal or better outcomes while using fewer resources.
Learning from these experiences is crucial to optimize recovery from the COVID-19 pandemic. We owe it to current and future cancer patients to use our combined learnings to make cancer care delivery more resilient to future disruptions and sustainability challenges.
LET’S DISCUSS
Inspired to share your thoughts? Or would you like to learn more on how to implement the learnings from the pandemic? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read the articles in our “Every day counts” blog series, which we published previously:
- White paper release: Every day counts
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second


