Improving regulatory timelines to improve time to patient access across Europe
The report “Every Day Counts – Improving Time to Patient Access to Innovative Oncology Therapies in Europe” established a collective understanding among stakeholders regarding the causes of delays in patient access to new cancer treatments across Europe. It focused on the stages following European Marketing Authorisation – Market Access and Patient Access – and included solutions and recommended next steps to reduce timelines for these stages.
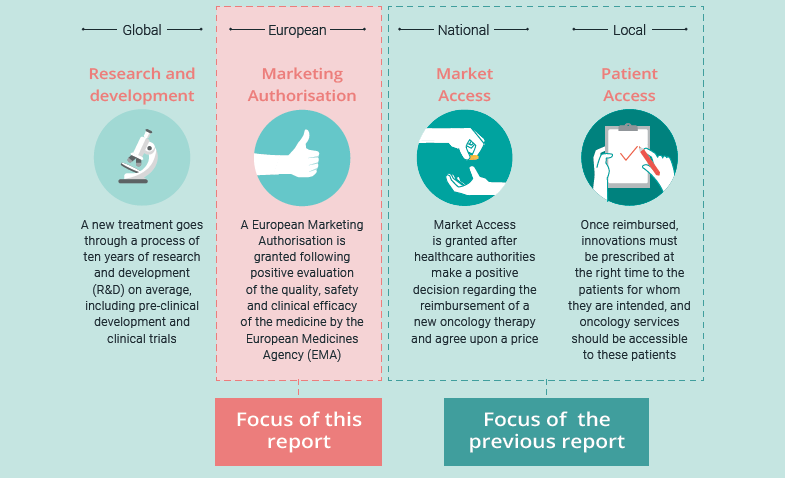

This follow-up initiative concentrates on the final stage of Marketing Authorisation: the administrative process between the final opinion of the Committee for Medicinal Products for Human Use (CHMP) and the final decision of the European Commission (EC). It aims to establish a clear and common understanding among European stakeholders of the steps involved in this part of the journey, and of potential opportunities to reduce timelines. This in turn will act as a basis for finding a common perspective on potential opportunities to accelerate the process.
The current process comes at a cost
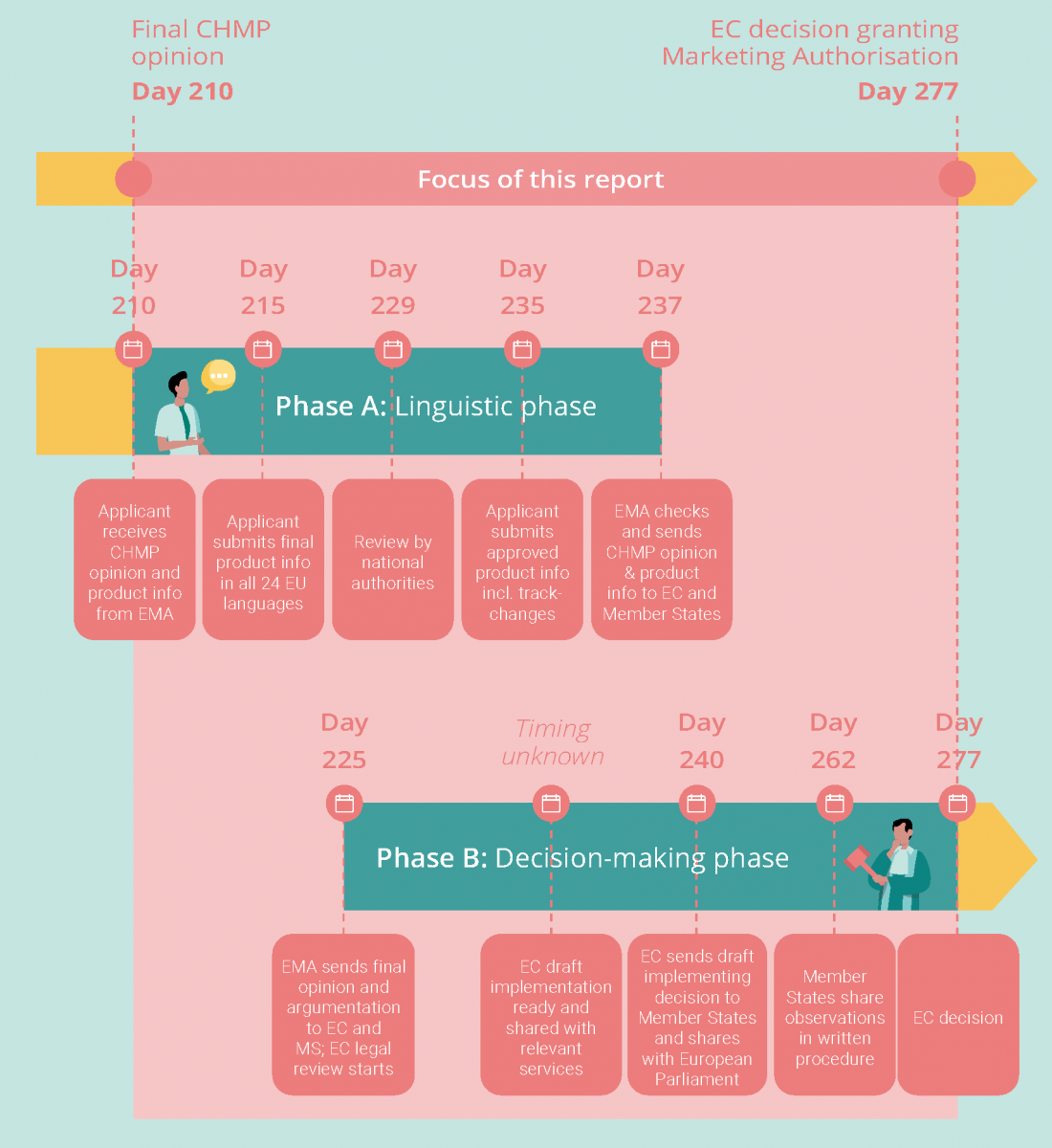
With a maximum official duration of 67 days (and a range of 33-198 days in practice), this administrative process represents only a small part of a medicine’s journey, but nevertheless an important opportunity for improvement. During the COVID-19 pandemic, it was expedited to less than one day for COVID-19-related vaccines. This suggests it is possible to shorten timelines for other approvals significantly, without affecting the quality and rigor of scientific review.
The report describes how current timelines come at a cost. Our illustrative analysis of 11 recently authorised oncology treatments, developed in collaboration with Swedish Institute for Health Economics (IHE), shows that the regulatory steps between CHMP opinion and EC decision together accounted for 18,600 years of potential life lost, although the full extent of life years lost is far greater when considering all oncology indications.

A once-in-a-generation opportunity
As the EC is currently considering changes to pharmaceutical legislation as part of the EU pharmaceutical strategy, we now have a once-in-a-generation opportunity for change. Even if only small improvements are made, they could have a significant impact on patients across Europe.
Three solutions to improves time to patient access across Europe
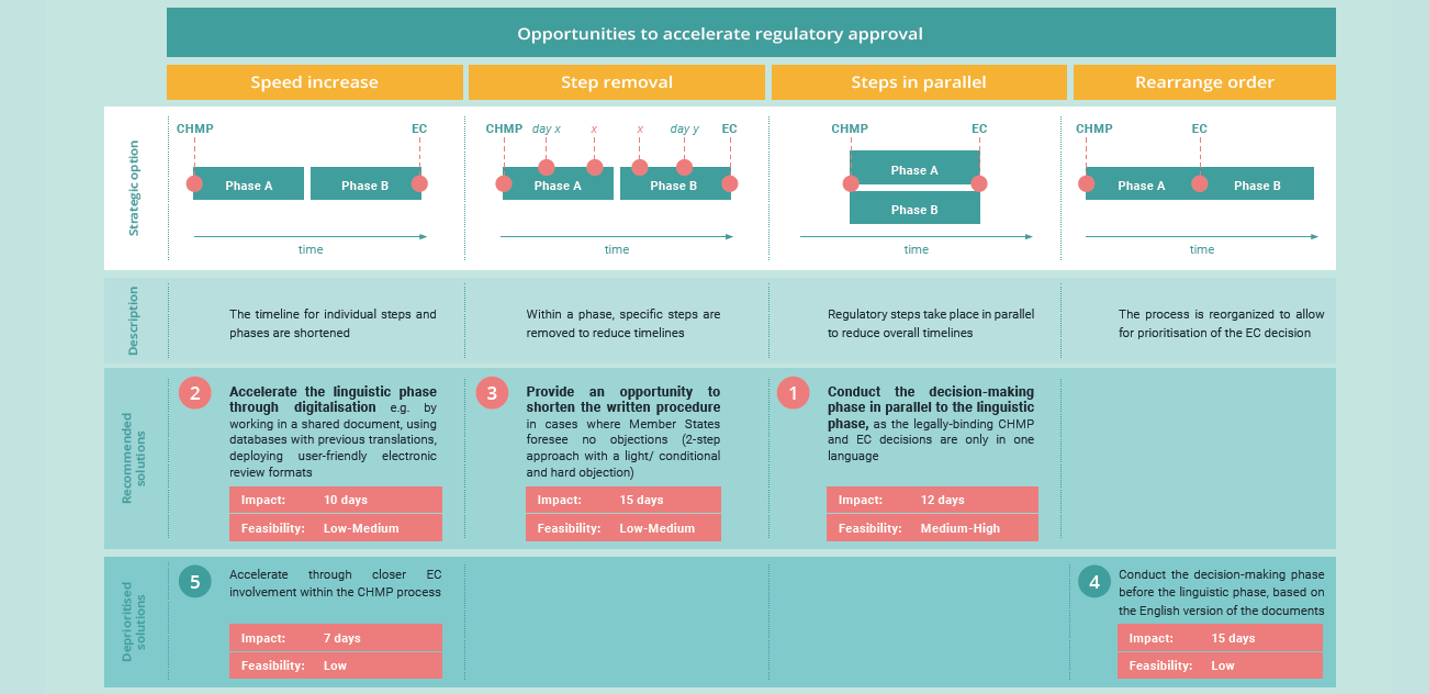

We describe three concrete solutions to be used as a basis for further dialogue among stakeholders. The three solutions have been developed on the basis of a review of the available literature, stakeholder interviews and two Sounding Board meetings with representatives from the EC, the European Medicines Agency (EMA) – including CHMP representatives, national regulatory authorities, national health technology assessment (HTA) bodies, professional healthcare associations, patient organisations, policy makers, payers, academic experts, and biopharmaceutical companies.
The project was initiated and financed by the EFPIA Oncology Platform (EOP).
The publication is endorsed by the following organisations:
- Acute Leukemia Advocates Network (ALAN)
- Associação de Enfermagem Oncológica Portuguesa (AEOP), Portugal
- Associação Melanoma Portugal, Portugal
- Associazione Contro il Melanoma (ACM), Italy
- Bulgarian Association of Clinical Research (BACR), Bulgaria
- Bulgarian Pharmaceutical Union (BPhU), Bulgaria
- Business School, Warsaw University of Technology (WUTBS), Poland
- Connaître et Combattre les Myélodysplasies (CCM), France
- Digestive Cancers Europe (DICE)
- European Association of Nuclear Medicine (EANM)
- European Cancer Patient Coalition (ECPC)
- European Federation of Pharmaceutical Industry Associations (EFPIA)
- European Union of Private Hospitals (UEHP)
- EVITA – Hereditary Cancer, Portugal
- Youth Cancer Europe (YCE)




