We identified ten reasons that explain most delays in giving European patients access to new cancer treatments. Here, our research focused on delays in ensuring market access and patient access. However, there is another access milestone preceding those two: marketing authorization. Let’s take a look at the overall picture of bringing new cancer treatments to European patients across all three access milestones.
Read more about the ten key factors delaying market access and patient access in Europe.
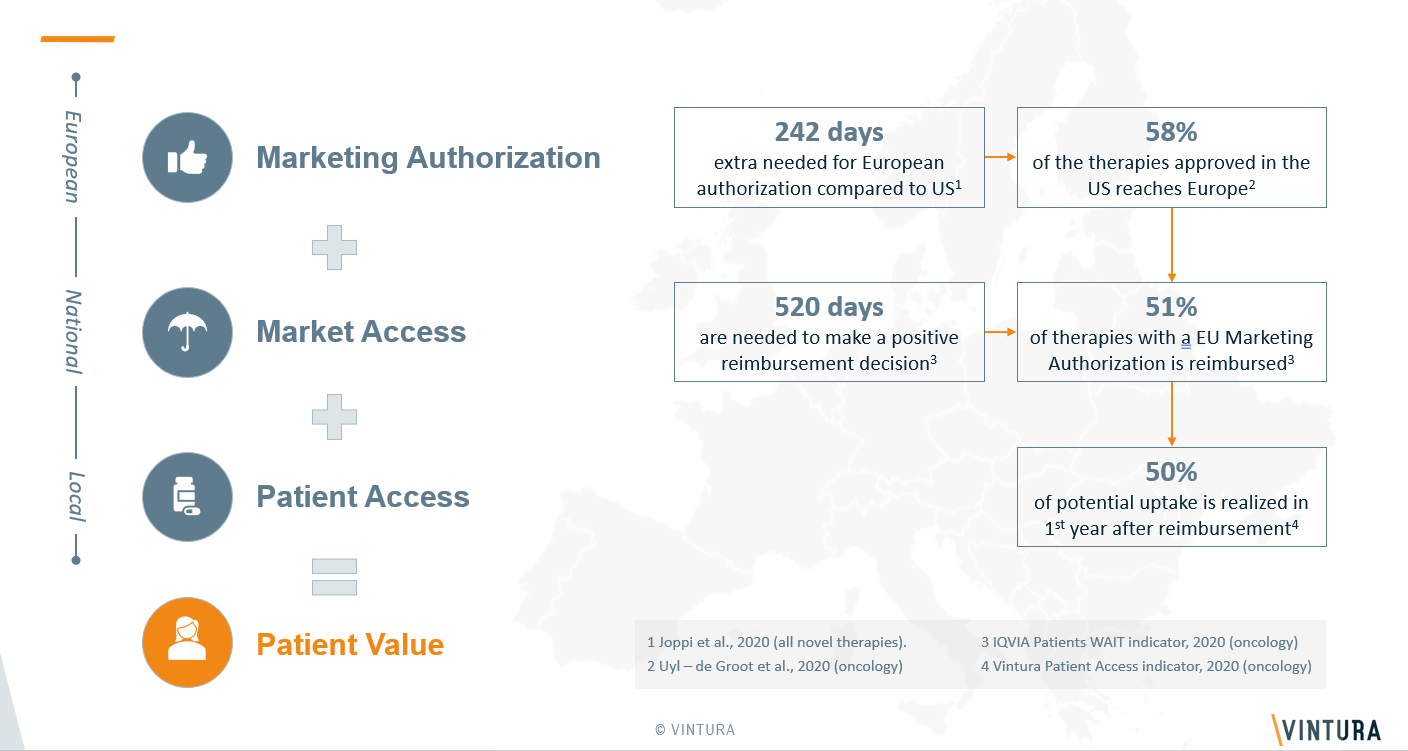

Milestone 1: Marketing authorization
First, a newly developed oncology treatment needs to be cleared by the European Medicines Agency (EMA). A European marketing authorization is granted when EMA positively evaluated quality, safety and clinical efficacy. On average, new cancer treatments achieve marketing authorization in Europe 242 days later than in the United States. Plus, of all FDA licensed therapies, only 58% is approved by EMA. As ensuring better access to new therapies is a joint responsibility, we call on both companies and EMA to do their part.
Milestone 2: Market access
Once cleared by EMA, national HTA bodies need to make transparent and evidence-based decisions on a treatment’s reimbursement, as these costs are public healthcare expenditures. In the case of oncology therapies, it takes on average 520 days for countries to reach a positive reimbursement decision. Of all therapies that receive a European marketing authorization, on average only 51% is reimbursed within the individual member states.
Learn how reducing time to Market Access dramatically impacts patients’ lives.
Milestone 3: Patient access
Once reimbursed, new treatments must be prescribed to the patients they are intended for, who must use them correctly. Among other things, this requires budget allocation. Apart from that, it’s vital to create clarity on the positioning of the new therapy in the treatment pathway by continuously updating clinical guidelines. Vintura’s Patient Access Indicator benchmark study identifies the actual prescription and use of new therapies in ten European countries. It shows that of all reimbursed therapies, only 50% of potential uptake is realized in the first year after reimbursement.
Learn about Vintura’s Patient Access Indicator benchmark study.
How to do better as a European community
When looking at the broader picture, we see that Europe faces serious access challenges across all three access milestones. On average, in Europe, it takes two years for a new cancer treatment to become available to a patient. Most treatments, however, are not available, either because those treatments are not registered (42%), or if registered are not reimbursed (49%), or if reimbursed are not applied in clinical practice (50%). The core issue here? Accountability.
Currently, none of the individual stakeholders is responsible for ensuring optimal timelines across all three access milestones, resulting in a sub-optimal process. As a European community, we could and should do better. Why? From a business perspective, we need to stay relevant as a market for innovation-minded investors. Yet most importantly, from a societal point of view, we want to make sure that the cancer patients within our community benefit from the latest developments in the field without any unnecessary delays. Because there is no value in developing innovative cancer therapies if patients can’t have access to them.
LET’S DISCUSS
Inspired to share your thoughts? Or would you like to learn more on how to accelerate patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Please also be invited to read our others articles in this “Every day counts” series:
- White paper release: Every day counts
- White paper Addendum: Every day counts – Addendum
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access



