A 3-year long administrative process before patients can access new cancer medicines
Innovative oncology therapies follow a long and winding road from laboratory to patients. The Research and Development (R&D) process typically takes around 10 years, followed by an administrative approval process which usually takes more than three years.
After R&D, there are three key milestones a candidate treatment must reach before it can receive full approval:
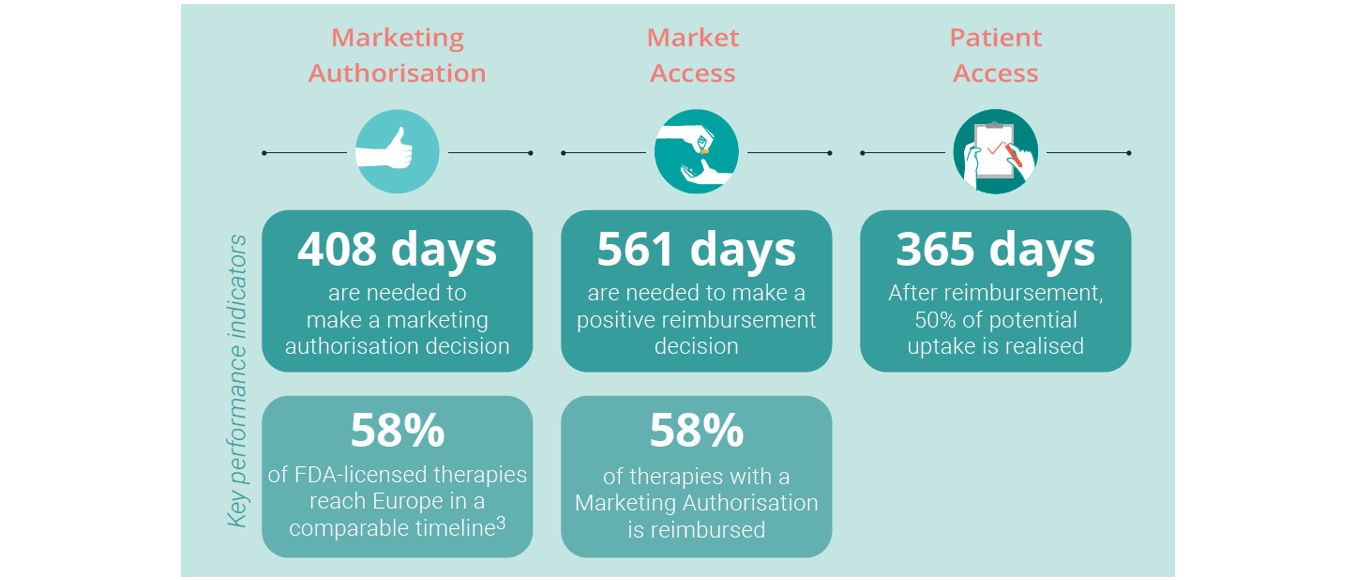
- Following an assessment process lasting a median 408 days the European Commission may grant a European Marketing Authorisation based on the opinion of the European Medicines Agency that the therapy is safe, effective and of high quality. This Authorisaton is required for the therapy to be used in EU and EEA countries.
- The next step is for national authorities to approve Market Access for the therapy. This refers to coverage under a reimbursement scheme to make the treatment financially accessible to the patient, and takes an average 561 days.
- Finally, Patient Access refers to the treatment being prescribed for and used by the patients for whom it is intended. This process can be delayed by, for example, medical guidelines not being updated to reflect medical, scientific or technological advantages. An analysis of 10 European countries found that one year after reimbursement, the average per capita use of innovative cancer medicines in Europe is only 50% of the average per capita use in the country in which the treatment is used most. Therefore, an important tranche of eligible patients are not being reached as fast as they could (Vintura, 2020).
As these milestones indicate, the approval process involves multiple stakeholders. The responsibility for optimising timelines and ensuring timely access for the maximum number of patients is shared between European and national policy makers, authorities, biotech companies, payers, and professional & patient organisations.

Current delays mean real costs to patients and society
Our analysis, conducted in collaboration with the Swedish Institute for Health Economics (IHE) and presented in our latest report in the series Every Day Counts, concludes that every 50 days of waiting for a therapy to be approved at the European level equates to more than 20,000 years of potential life across Europe. This corresponds to a productivity loss of more than €130 million across the European economy.
Starling though it is, this impact assessment is based on just 11 oncology treatments and indications. The actual impact is likely to be far greater, as it would include:
- All (oncology) therapies
- The benefit of improved quality of life, rather than simply life years gained
- Productivity losses related to sickness, rather than just death
- Informal productivity gains rather than just formal productivity gains.
Nevertheless, the 20,000 year figure for every 50 days shows more clearly than ever that for oncology patients, every day really does count, and the important gains that can be made when we are able to expedite the process of bringing innovations to patients.
The European community must do better
While the processes which lead to the three milestones listed above are robust, the fact that no individual stakeholder is responsible for ensuring optimal timelines across the whole process means it is currently sub-optimal.
As a European community, we could and should do better. On a societal level we have an obligation to ensure that cancer patients across Europe can benefit from the value offered by the latest treatments and innovations as soon as possible. On an economic level we should make every effort to minimize productivity losses linked to sickness and avoidable death, and on the business level Europe needs to remain a relevant market for innovation-minded investors. A shorter, more optimal approval process would be a key step towards delivering on all these fronts.
Multi-stakeholder collaboration is key
Reducing time to patient access is an important step in maximising the value to patients offered by a new treatment. It is a worthwhile goal: achieving it is the joint responsibility of every stakeholder. Those stakeholders should co-operate to achieve a shared understanding of how they can collaborate in order to reach it.
To that end, Vintura was engaged by the EFPIA Oncology Platform to establish and develop a clear and common understanding among European stakeholders of the steps involved in journey from laboratory to patient, and to identify potential opportunities to reduce the time each of these steps takes.
Three concrete opportunities to shorten these timelines are described in this latest report.
Real opportunities to deliver more value, sooner
For this third report we worked in close collaboration with all relevant stakeholders, it puts a particular focus on two aspects of the administrative process between the final opinion of the Committee for Medicinal Products for Human Use (CHMP) and the final decision of the European Commission (EC).
Three concrete opportunities to shorten timelines associated with this process are described in the report.
This report complements the report ‘Every Day Counts: Improving time to patient access to innovative oncology therapies in Europe’, which focus on the next phase of Market Access and Patient Access. In this report Vintura identified 10 key factors in delaying patient access, including delays caused by process, by reimbursement criteria and the readiness of health systems to integrate new treatments in their clinical practice.
Both these reports serve as an invitation for further dialogue and joint action between stakeholders. Because for cancer patients, Every Day Counts.
Let’s discuss
Inspired to share your thoughts? Or would you like to learn more on how to work together effectively with multiple stakeholders to shorten times to patient access? We would be delighted to hear from you. Please feel invited to contact Bas Amesz.
DO YOU WANT TO READ MORE?
Here are all the articles in the “Every day counts” series
- White paper release: Every day counts – Improving time to patient access to innovative oncology therapies in Europe
- Blog: Bringing stakeholders together to improve patient access to oncology therapies in Europe
- Blog: European access discussions shouldn’t stop at reimbursement
- Blog: Deep dive: The Patient Access Indicator
- Blog: The 10 key factors delaying patient access across Europe
- Blog: Introducing new cancer treatments: how reducing time to Market Access dramatically impacts patients’ lives
- Blog: Speeding up patient access requires dealing with uncertainty on clinical value first, negotiating price second
- Blog: Outdated clinical guidelines prevent timely adoption of oncology innovations into medical practice
- Blog: Europe’s patchwork of evidence requirements is an important factor in delayed patient access
- Blog: Bringing new cancer treatments to Europe: the challenge of making it across all three access milestones
- White paper release: Every day counts –The impact of COVID-19 on patient access to cancer care in Europe.
- Blog: How COVID-19 has affected European patients’ access to cancer care, and what we can learn from the crisis
- Blog: COVID-19 has hurt our ability to reduce cancer deaths
- Blog: 6 learnings from the COVID-19 crisis to make cancer care delivery in Europe more resilient to future challenges
- White paper release: Every day counts – Improving regulatory timelines to optimise patient access to innovative oncology therapies in Europe




