This paper aims to analyse and demonstrate the unique nature and value of PDMPs (Plasma-derived Medicinal Products) across clinical, economic, and societal dimensions, and focuses on improving Patient Access. Patient Access is viewed from two angles: formal access based on reimbursement coverage, and therapeutic access based on the availability of an optimal treatment paradigm. It also analyses key challenges that affect the full realisation of the value of PDMPs. Finally, it offers a comprehensive view of possible solutions to the identified challenges.
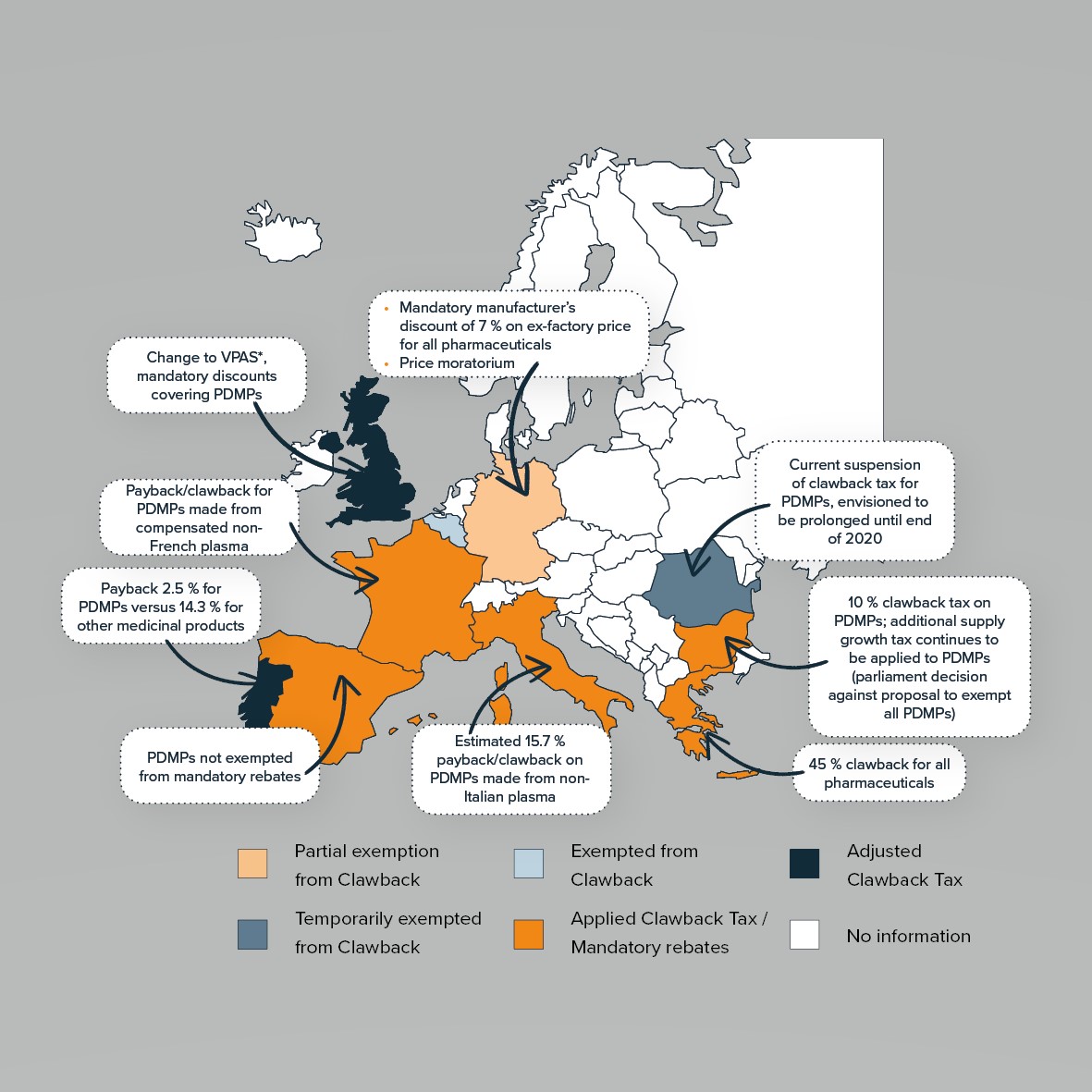
PDMPs are unique biological therapies derived from human plasma and are used to treat patients with rare, often genetic conditions with a high disease burden. Despite decades of effective therapeutic use in Europe, and demonstrable clinical and societal value, these treatments still face numerous Patient Access challenges pertaining to the plasma donation landscape, regulatory and reimbursement frameworks, and treatment paradigms.
There is a growing clinical need of European patients for PDMPs, and considerably more plasma must be collected in Europe. As new indications arise more patients are diagnosed with diseases requiring PDMP treatment. Even when diagnosed and if therapy is available, patients often are denied adequate PDMP treatment because of therapeutic and formal Patient Access challenges. To overcome these challenges, it is necessary to form close and trust-based partnerships between industry and all healthcare stakeholders.
Read all about it here, we are looking forward to hearing your thoughts on this topic!
> Download the Report here <
LEARN MORE
Do you want to share your thoughts with us on Patient Access to Plasma-derived Medicinal Products? Do you want more information or want to get started? Please contact Silvia Rohr.