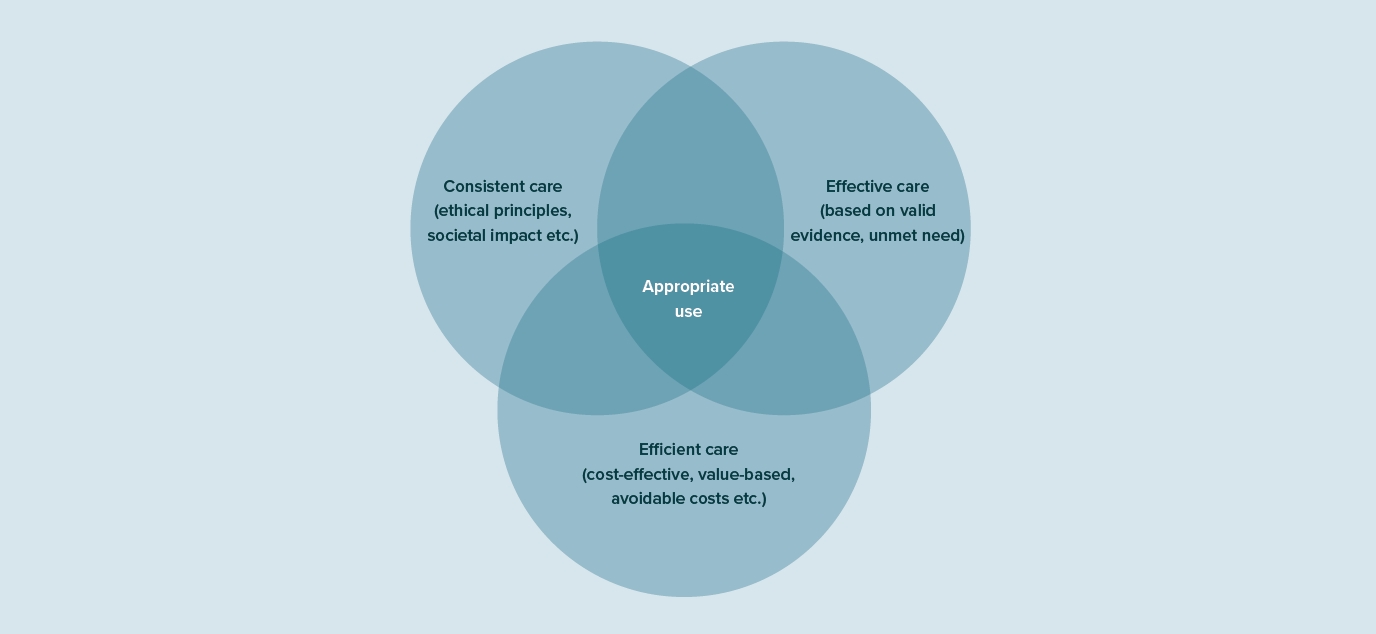
Our green paper is the result of an iterative process with two Advisory plenary meetings and a series of individual interviews. The Advisory Committee was comprised of leading European clinicians from different medical fields and unified by their expertise in the therapeutic use of IgGs across a wide range of severe and often life-threatening conditions and diseases. Our green paper explores possible ‘appropriate use’ frameworks, supported by evidence of clinical benefits, expanded through contributions from the Advisory Committee.
The Advisory Committee have identified two sets of Preliminary Recommendations:
1. ADDRESS THE ROOT-CAUSE OF IgGs’ AND PLASMA’S LIMITED AVAILABILITY
-
- RESILIENCE: explore key conditions and actions necessary to ensure resilience of European healthcare systems, both in terms of increasing plasma collection volumes and ensuring IgGs’ availability for appropriate therapeutic use.
- EDUCATION: build awareness through local and pan-European public health education, tailored to all ages, promoting regular plasma donation initiatives.
- INFRASTRUCTURE: invest in modern infrastructure and expertise for efficient plasmapheresis delivery, alongside all effective measures for boosting source plasma donations, including coexistence of private and public systems.
2. RE-ASSESS AND ENABLE ACCESS TO APPROPRIATE IgG USE
-
- RE-ASSESSMENT: systematically reassess and acknowledge growing medical need for IgGs to ensure equitable access for all patients who will benefit in line with the appropriate use framework.
- ACCESS: review regulatory requirements to authorise appropriate IgG use in line with the proposed framework criteria and evidence scale.
- EVIDENCE: intensify efforts to generate and analyse high quality clinical trial and real-world data across rare immune-mediated diseases and secondary immune deficiencies.
- COLLABORATION: stimulate pan-European collaboration to exchange best clinical practices and data and foster joint initiatives, studies, and registries, at both the single indication level and cross- indication level.
- PATIENT VOICE: ensure patients and patient advocacy groups are educated and meaningfully involved in the discussion on IgG therapeutic value and the future of the IgG therapeutic use in Europe.
Download our green paper to learn more about this subject and deep dive into how to evaluate the growing medical need & demand vs. the finite availability of plasma, based on the optimal and appropriate use of IgGs. If you have questions, please reach out to Natalia Eitel.